
How to calculate valency of 1st 20 elements YouTube
The valency of the first 30 elements of the periodic table is given below. Periodic Trends in the Oxidation States of Elements 1. Variation Of Oxidation State Along a Period While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8.

Easy way to memorize the Valency of first 20 Elements. YouTube
This video will help students to understand electronic configuration, valence electrons and valency.For more videos on Physics, Chemistry and Mathematics ref.

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Elements Their Atomic
The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined - 4s 2, 4p 1). The completely filled d orbitals count as core, not valence, electrons. Transition elements or.

What Are the First 20 Elements Names and Symbols
1 Answer Stefan V. Jan 13, 2015 So, the first 20 elements in the periodic table start with H and end with Ca. The quickest way to remember the number of valence electrons is to form a relationship with the number of the group the element is located in.
First 20 Elements Of The Periodic Table With Atomic Number And Mass Valency Review Home Decor
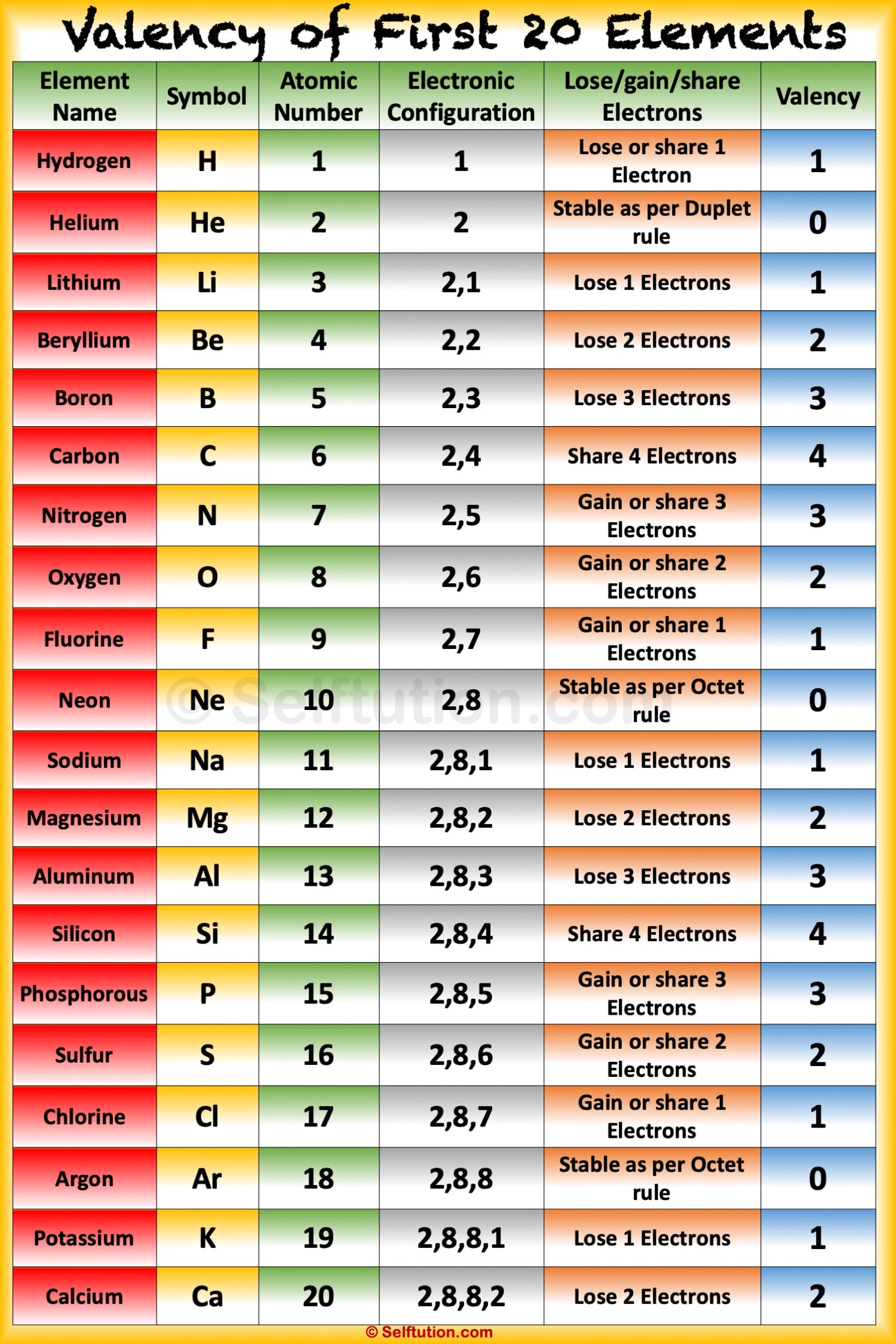
The valency of elements from 1 to 20 showcases some fascinating chemical behavior. Here are the valencies of selected elements: Hydrogen (H): Hydrogen typically exhibits a valency of +1, forming compounds like H2O (water) and HCl (hydrochloric acid).

Periodic Table With Names And Atomic Mass Number Valency Awesome Home
1.8K Share 146K views 2 years ago The Periodic Table In less than 2 minutes, you'll remember the valency of elements 1 - 30! We'll use 2 valency tricks - valency for the first 20.

Periodic Table Of Elements With Atomic Mass And Valency
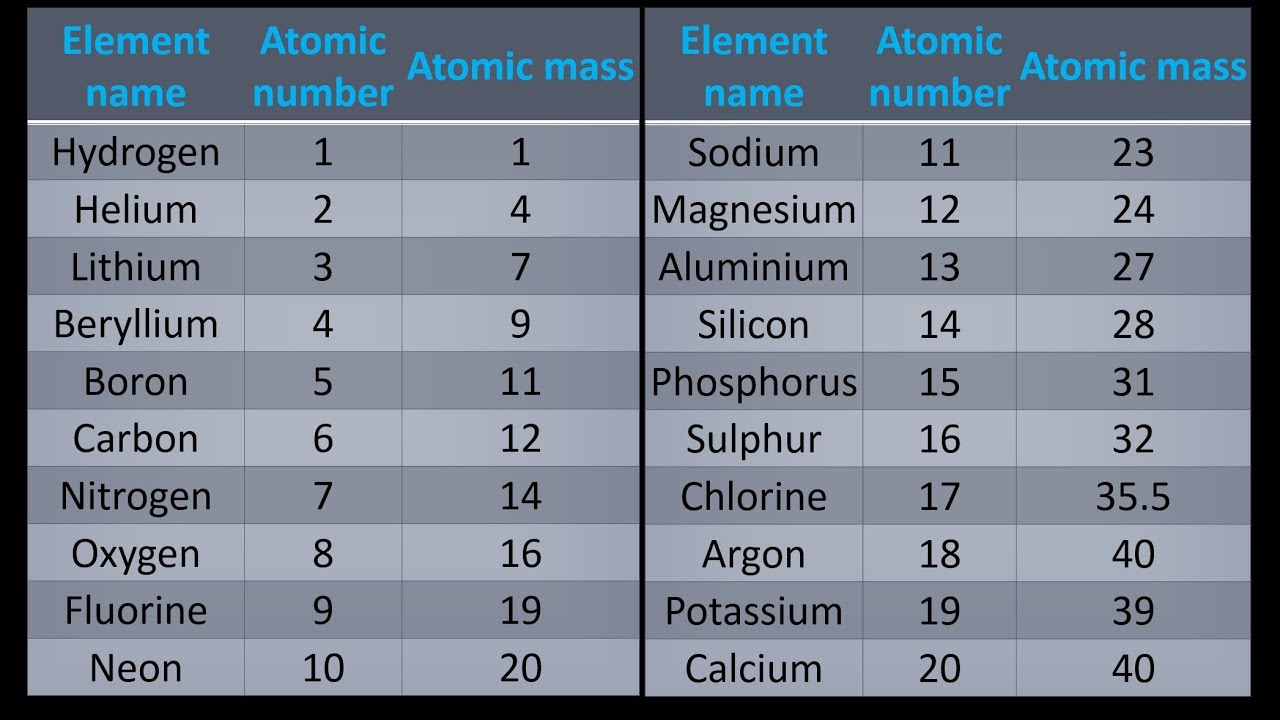
List of the First 20 Elements The elements are listed in order of increasing atomic number. The atomic number is the number of protons in atoms of each element. The first 20 elements and their symbols are: Hydrogen (H) Helium (He) Lithium (Li) Beryllium (Be) Boron (B) Carbon (C) Nitrogen (N) Oxygen (O) Fluorine (F) Neon (Ne) Sodium (Na)

periodic table of elements printable flashcards chemistry etsy find various types of valency
Periodic Table -. The periodic table of elements graphic is the most common tool to utilized to compute valency in this method. All of the metals in column 1 have a valency of +1, for example, hydrogen, lithium, sodium, and so on. Similarly, all of the elements in column 17 have valency 1, including fluorine, chlorine, and other elements.

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Do some elements have
Get essential facts about the first 20 elements, all in one convenient place, including the name, atomic number, atomic mass, element symbol, group, and electron configuration. If you need detailed facts about these elements or any of the higher numbered ones, start with the clickable periodic table . 01 of 20 Hydrogen davidf / Getty Images

chemistry first 20 elements table with electronic configuration and valency Brainly.in
This table of element valences includes the maximum valence and most common valence values. Use this is a reference with a periodic table.

Periodic Table Elements Valency Charges Elements เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 1465162751
What is the reason for this particular formula? The answer to the above question is "Valency". Let us know more about Valency and how it helps in determining a formula! Suggested Videos What is Valency? Valency is the measure of the combining capacity of atoms or molecules.
A Simple Way To Get Atomic Mass Of First 20 Elements Of The Periodic Free Hot Nude Porn Pic
Easy way to memorize the Valency of first 20 Elements. Chemistry Tutor HR 659 subscribers Subscribe 417 views 2 years ago PERIODIC TABLE This is a trick to learn the valency of first 20.

A simple way to understand and memorise the valency of first 20 elements of the periodic table
Valency of first 20 elements in tabular form Electronic Configuration The arrangement of electrons in the various shells of an atom of the element is called electronic configuration. The maximum electrons which can be accommodated in K shell are 2, for L shell is 8, for M shell is 18 and for N shell is 32.
Valency and Variable Valency Valence Shell and Electrons » Selftution
Solution Valency: "The electrons present in the outermost shell of an atom are known as the valence electrons." "Valency is the combining capacity of an atom." The valency and valence electrons for the first 20 elements are discussed below: Suggest Corrections 953 Similar questions

Valency of the elements from 1 to 30 Science Materials Metals and NonMetals 13851567
These are the first 20 elements, listed in order: H - Hydrogen He - Helium Li - Lithium Be - Beryllium B - Boron C - Carbon N - Nitrogen O - Oxygen F - Fluorine Ne - Neon Na - Sodium Mg - Magnesium Al - Aluminum Si - Silicon P - Phosphorus S - Sulfur Cl - Chlorine Ar - Argon K - Potassium Ca - Calcium Element Symbols and Numbers

Valency Trick Trick to find valency of 51 to 60 elements,Trick to learn valency of 51 to 60
First 20 Elements The first 20 elements of the periodic table have been tabulated below, along with their symbols and atomic numbers. Learn more ⇒ Interactive Periodic Table Table of Contents What Information does the Atomic Number of an Element Provide? Why is Potassium denoted by the symbol 'K' and Sodium by the symbol 'Na'? Recommended Videos